The NuVasive MAGEC is a rod implant system used in children for the treatment of early-onset scoliosis. NuVasive recently recalled the MAGEC system after it was discovered that a defect was causing the endcaps on the implant rod to detach inside the body. Injuries from this defect include back pain, bone abnormalities, and inflammation. Individuals who have been harmed by a defective MAGEC system rod are now filing product liability lawsuits against NuVasive.
About the NuVasive MAGEC System
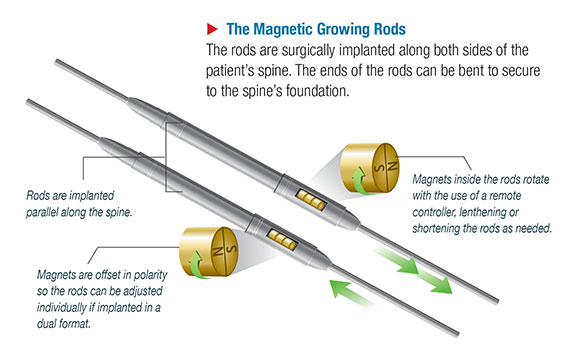
NuVasive is a medical device company focused on the development and manufacture of high-tech spinal surgery implants. The NuVasive MAGEC system is a magnetic rod system that can be surgically implanted in the spine for the treatment of early-onset scoliosis (EOS) in children. The MAGEC system features a growing rod with innovative magnetic technology that enables the rod to be adjusted eternally with a remote control.
Traditionally, treatment of EOS involved surgically implanting a fixed rod in the spine. As the child grew, the fixed rod would periodically have to be surgically removed and replaced with a bigger rod. The external adjustment capability of NuVasive’s MAGEC rod was a major leap forward in this process. The MAGEC enables doctors to adjust the size of the rod externally with a remote thereby eliminating the need for repeated surgeries.
NuVasive MAGEC Safety Recall
In February 2020, NuVasive issued an emergency recall of all its MAGEC devices. The emergency safety recall was prompted by the discovery of the design defect involving a potentially dangerous mechanical component failure in the MAGEC adjustable growing rod.
The defect was causing the endcap on the MAGEC lengthening rod to break off and separate from the end of the rod. This end cap separate defect was reportedly occurring in 1% of implanted devices. In July 2020, NuVasive received formal approval from the Food and Drug Administration (FDA) for a modified design of the MAGEC adjustable rod that would mitigate the defect causing the endcap to separate.
Following the initial safety recall of the MAGEC system, new concerns began to emerge in December 2020 about potential biocompatibility complications arising when defective end caps separated from the MAGEC rod. This prompted NuVasive to issue an updated Field Safety Notice about the MAGEC defect and potential complications.
Beginning in early 2021, the FDA also began to receive reports that separation of the endcap on NuVasive MAGEC rods was causing inflammatory reactions in local tissue around the device. This prompted NuVasive to place all MAGEC devices on a global shipping hold pending further review. The FDA is continuing to work with NuVasive to assess the potential risk of this biocompatibility problem and whether there is any “clinically meaningful impact to patients with MAGEC devices.”
Which MAGEC Systems Were Recalled?
NuVasive’s emergency safety recall covered the following MAGEC system devices:
- MAGEC System Model X
- MAGEC System Rods
- MAGEC Spinal Bracing and Distraction System
- MAGEC 2 Spinal Bracing and Distraction System
The modified version of the MAGEC system was approved by the FDA and released back onto the market in July 2020. However, new concerns have emerged about whether the end cap components are biocompatible inside the body. This new issue is currently being examined by the FDA and it could prompt another recall.
Potential Injuries Related to the NuVasive MAGEC Defect
The defect with the MAGEC involving detachment of the endcap from the rod can potentially cause a number of serious adverse health conditions for patients. The biocompatibility problems with the endcap component could also result in various complications. Injuries and complications associated with these defects in the MAGEC system could include:
- Bone abnormalities
- Cancer
- Soft tissue inflammation
- Pain and irritation
- Tissue damage or discoloration
NuVasive MAGEC Recall Lawsuits
Anyone who had the NuVasive MAGEC rod system surgically implanted for the treatment of EOS can potentially file a product liability lawsuit against NuVasive if that MAGEC system caused serious health complications. Anyone who had the MAGEC device implanted after September 2014 would potentially be eligible.
Compensation for NuVasive MAGEC Rod System Defects
If your child had the NuVasive MAGEC rod system surgically implanted for their EOS and that system failed or caused complications including, but not limited to, separation of the end cap from the rod, you may be entitled to financial compensation for any resulting injuries.
Miller & Zois is a national product liability law firm that handles defective medical device cases. Contact our offices today to find out if you might be entitled to compensation for your NuVasive MAGEC related injuries.
 Lawsuit Information Center
Lawsuit Information Center