Prilosec and Nexium kidney damage class action lawsuits are still being filed around the country.
Thousands of Proton Pump Inhibitor lawsuits have been filed around the country by plaintiffs who allege that PPI drugs like Nexium and Prilosec caused them to develop permanent kidney damage, bone fractures, and interstitial nephritis. As of August 2023, there are nearly 13,000 Nexium and Prilosec kidney damage lawsuits pending in a class action MDL. Settlement rumors in this litigation are swirling.
September 2024 Parkinson’s PPI Link?
In a recent report published in this month’s issue of JAMA Network Open, researchers found that patients with damage to the lining of the small intestine, esophagus, and other gastrointestinal areas face a 76% higher risk of developing Parkinson’s disease. This risk is particularly elevated among individuals who use heartburn medications like Prilosec (omeprazole) and Nexium (esomeprazole), as well as popular pain relievers such as Advil (ibuprofen), Motrin (ibuprofen), and Aleve (naproxen).
The study further highlighted that nonsteroidal anti-inflammatory drugs (NSAIDs) and proton pump inhibitors (PPIs), often used for pain relief and acid reflux, contribute to gastrointestinal damage, which may act as a trigger for the onset of Parkinson’s disease. Researchers emphasized the need for close monitoring of patients with these GI issues, especially those using these common medications, as early intervention could help reduce future Parkinson’s risk.
August 2023 PPI Case Count
There are 12,833 lawsuits now in MDL 2789, making the PPI MDL the fifth biggest MDL in the country. There are settlement rumors out there. But we have all heard that one before in this litigation. There was a settlement conference in New Jersey that was set for August 14th before Magistrate Judge Leda D. Wettre. But it was postponed due to “unavoidable scheduling conflicts.”
June 2023 Bellwether Trial Update
The first Nexium lawsuit over kidney damage warnings, which was scheduled to begin in March as a bellwether trial as settlement rumors continue to swirl. The trial is now scheduled to commence on June 5, 2023. Again, the plaintiff in this first trial is James Rieder, a Nexium user who developed chronic kidney disease after using the drug for nearly five years. The likelihood now is a global PPI settlement to resolve most PPI lawsuits before this case goes to trial.
The court issued an order this month for lawyers for the Plaintiff Leadership Committee and the Takeda/Abbott defendants to meet for a settlement conference before Magistrate Judge Leda D. Wettre on August 14, 2023. The court ordered that the parties come with full and immediate settlement authority.
January 2023 Class Action Lawsuit
As the hope of global settlement grows, Nexium/Prilosec lawsuits continue to be filed. Right after the new year, Bernstein Liebhard filed Levy v. AstraZeneca Pharmaceuticals in the MDL in New Jersey. In this case, a Tennessee man alleges he took Nexium and Prilosec and suffered injuries that required him to need dialysis.
September 2022 Class Action Update
The opening bellwether test trial is set for October and will feature Plaintiffs James Reider (James Reider v. AstraZeneca Pharmaceuticals LP, et al. – 2:19-cv-00850).
Last month, the defense filed several pre-trial motions in Reider which seek to exclude or limit testimony from certain plaintiff witnesses at trial.
The Nexium class action judge will hear these motions on September 19, not long before the trial which is when these types of motions are usually ruled on by the judge. The result of the Reider trial will have a major impact on the future course of this PPI class action lawsuit and will impact future settlement amounts. Two more bellwether PPI trials will be held next year.
August 2022 Class Action Update
Defendants in the Proton Pump Inhibitor (Nexium/Prilosec) MDL have argued that the plaintiffs’ failure to warn claims should be dismissed under the federal preemption doctrine because the FDA approved the warning labels for the drugs. This is a critical argument. If defendants were to win on this issue, the PPI lawsuits involving Nexium and Prilosec would effectively end.
But, thankfully, that will not happen. On Monday, Special Master Ellen Reisman issued a report and recommendation to the MDL Judge advising that this preemption argument should be rejected. Reisman noted that the argument should fail because the defendants cannot show that the FDA would have rejected a proposal to include stronger warnings about kidney injuries.
The first bellwether Nexicumtest trial in the MDL is set for November with five more to follow.
July 2022 Class Action Update
The first big Nexium-Prilosec PPI lawsuit in the MDL class action is heading for trial in November. Plaintiffs’ lawyers defeated an effort by Merck and AstraZeneca to get out from under the lawsuit. The court rejected the defendants’ motion for summary judgment on the failure to warn claim – the key claim in James Rieder’s Nexium kidney injury lawsuit. So it is all systems go for trial this fall.
June 2022 Class Action Case Count
There were 13,366 pending cases in the Prilosec/Nexium Proton Pump Inhibitor MDL as of July 15. This is actually less than 13,437 cases that were pending in the PPI MDL as of June 15. The reduction comes after a large group of cases were voluntarily dismissed by the plaintiffs and only a few new cases were transferred in. Meanwhile, we are just four months away from the first bellwether test trial set to begin in November.
May 2022 Class Action Update
Defense lawyers ave been filing motions to dismiss the Prilosec and Nexium lawsuit bellwether cases in the Nexium-Prilosec PPI MDL. The gist of these motions is that preemption. This is the idea that federal law preempts victims from being able to file lawsuits under state tort laws. . A new decision on May 23, 2022 from the District of New Jersey (In re Fosamax Alendronate Prod. Liab. Lit., could impact the federal preemption issue. The Special Master, who assists the judge administer pre-trial discovery in the Nexium-Prilosec class action lawsuit, issued CMO No. 75. This order allows the lawyers to offer their positions on how this decision might impact a Prilosec or Nexium lawsuit. (I don’t think this case – which rules for the defendant – has any impact on the issues in the Nexium class action lawsuit.)
April 2022 Class Action Lawsuit Update
If the initial round of bellwether trials in the Nexium-Prilosec PPI class action MDL does not generate a global settlement, the remaining 13,500 cases will be remanded for trial in large groups of several hundred. This would put great pressure on lawyers on both sides because this is a lot of PPI lawsuits that need to be prepared for trial. To effectuate plan, Judge Wolfson issued CMO #74. This order identifies a group of 200 “Wave One” cases in which the parties will start preparing for trial over the upcoming months. This will be followed by additional waves, each consisting of several hundred cases.
Proton Pump Inhibitors (Nexium and Prilosec)
Drugs in the family known as proton-pump inhibitors (“PPIs”) are primarily used in the treatment of gastroesophageal reflux disease (“GERD” or “acid reflux disease”). PPIs are one of the most commonly prescribed medications in the U.S. with over 15 million prescribed users. PPI drugs work by reducing hydrochloric acid in the stomach.
Nexium and Prilosec are two of the leading brands of PPI drugs. Both Nexium and Prilosec are manufactured by AstraZeneca. Nexium is one of the biggest selling drugs in the world with annual revenues of over $5 billion.
Nexium and Prilosec Linked to Chronic Kidney Damage
Since PPIs like Prilosec and later Nexium was first introduced on the market back in the 1990s, several scientific studies have linked long-term PPI use to serious adverse health effects such as bone fractures and pneumonia. In 2015, however, new evidence emerged that conclusively showed that prolonged use of PPIs could significantly increase the risk of permanent kidney injuries.
Recent studies have shown the long-term use of PPIs is independently associated with a 50% higher risk of chronic kidney disease (“CKD”), even after adjusting for several potential confounding variables, including demographics, socioeconomic status, clinical measurements, and prevalent comorbidities, and concomitant use of medications. In one of those studies, the use of PPIs even for a very short period was shown to increase the risk of CKD by 10%.
CKD (chronic kidney failure) is a condition involving the gradual loss of kidney function. Kidneys filter waste and excess fluid from the blood, which is then excreted. When chronic kidney disease reaches an advanced stage, dangerous levels of fluid, electrolytes, and waste can build up in the body. CKD can progress to end-stage kidney failure, which is fatal without artificial filtering, dialysis, or a kidney transplant. CKD is associated with a substantially increased risk of death and cardiovascular events.
Nexium and Prilosec Kidney Damage Lawsuits
Since 2016, a growing wave of PPI kidney damage lawsuits has been filed against AstraZeneca and other PPI manufacturers. To date, over 15,000 plaintiffs have filed PPI kidney damage lawsuits against the manufacturers of Nexium, Prilosec, Prevacid, Protonix, and Dexilant.
The PPI lawsuits allege that AstraZeneca and other manufacturers knew or should have known about the correlation between the use of PPIs and the significantly increased risk of chronic kidney disease and acute kidney injuries. Despite clear knowledge that PPIs like Nexium and Prilosec caused CKD and acute kidney injuries, the lawsuits accuse PPI manufacturers of continuing to market and sell their drugs without warning consumers or healthcare providers of the significant risks of kidney disease or injury.
PPI Class Action MDL 2022 Update
The very first PPI kidney damage lawsuit was filed in 2016 by a plaintiff who claimed that he developed kidney disease after using Nexium from 2013 to 2015. The number of PPI kidney damage lawsuits filed across the country slowly increased over the first two years as more people and doctors became aware of the evidence linking PPIs to kidney damage.
By August 2017, several thousand PPI kidney damage lawsuits were pending in federal courts around the country. The Judicial Panel of Multidistrict Litigation (JPML) consolidated the cases into a new PPI MDL (Proton-Pump MDL 2789). The Proton-Pump class action MDL was assigned to judge Clair C. Cecchi in the District of New Jersey.
As of March 2022, 13,470 active PPI cases were pending in the MDL. Thousands more cases are expected to be added as new PPI kidney damage lawsuits are still being filed.
The opening round of bellwether test trials in the PPI MDL is set to begin in October 2022. There will be three bellwether trials in the first round, all held in New Jersey and all featuring plaintiffs who used Nexium, Nexium, or Prilosec. The results of these initial bellwether trials will have a major impact on the course of the litigation moving forward.
- Truvada kidney injuries are also the subject of litigation
Who Qualifies for a PPI Kidney Damage Lawsuit?
Anyone who regularly took a proton pump inhibitor in prescription or over-the-counter form for at least 12 months and was subsequently diagnosed with kidney damage or chronic kidney disease may be eligible to file a PPI lawsuit. Common brand name PPI drugs include Nexium, Prilosec, Dexilant, Prevacid, and Protonix.
If you developed kidney damage or were diagnosed with chronic kidney disease or kidney failure after using one of these drugs for an extended period, you may be entitled to financial compensation. Although thousands of PPI kidney damage lawsuits have already been filed, it is not too late to file your lawsuit.
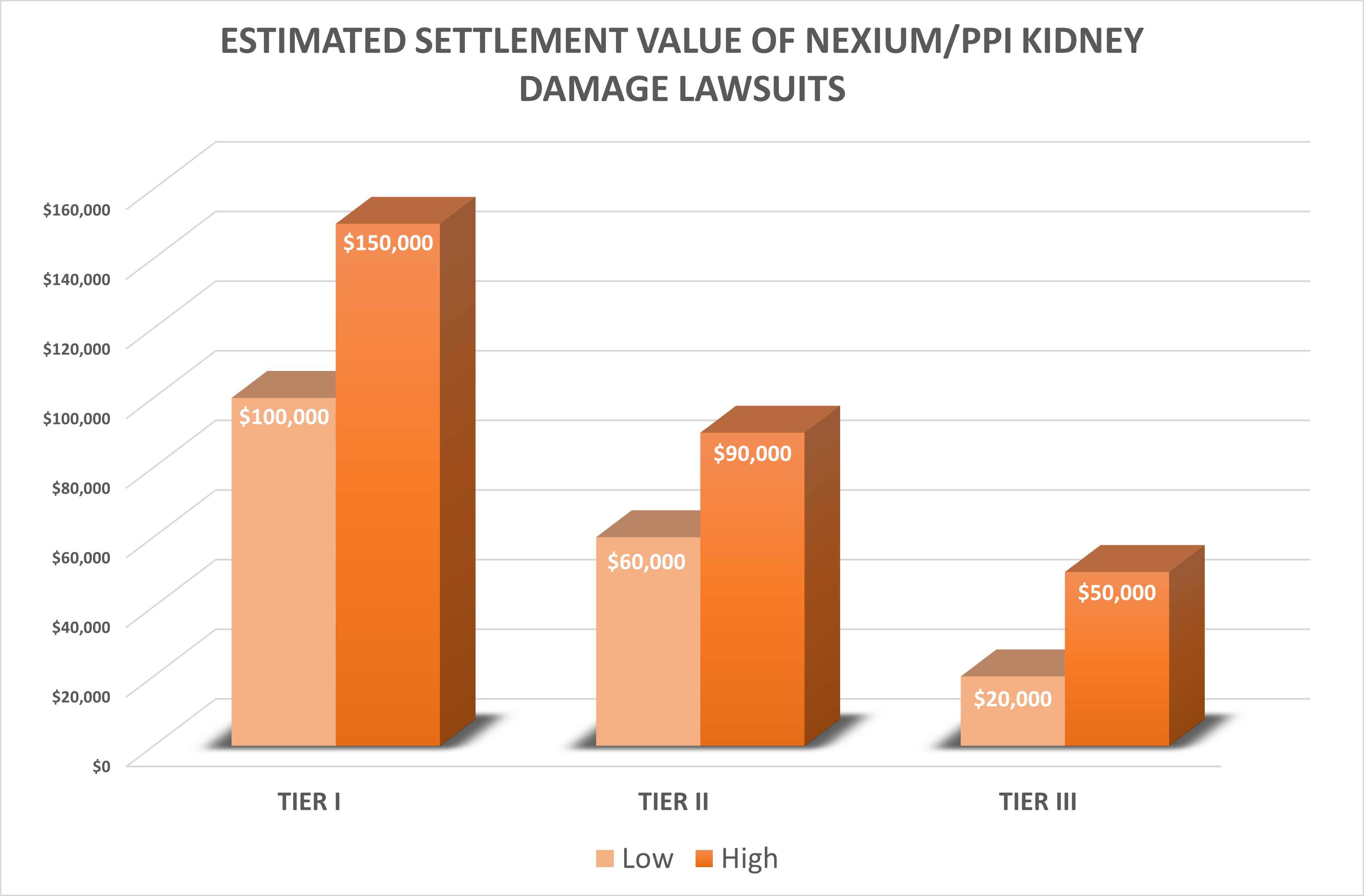
How much will Nexium kidney damage lawsuits be worth?
Our best estimate is that Nexium or PPI kidney damage lawsuits in the top settlement tier will have a settlement value around $100,000 to $150,000. Cases in the lowest tier may be worth $20,000 to $50,000.
Estimated Settlement Value of PPI Kidney Damage Lawsuits
There have not been any reported settlements or verdicts in any PPI lawsuits alleging kidney damage, so we can’t say for certain what the likely settlement value of these cases will be. But there is no daylight between our projected Nexium settlement amounts and Prilosec and other PPIs. But settlement rumors are swirling in October 2022.
So, again, based on results in prior kidney damage or failure cases coupled with our Nexium lawyers’ overall take on the litigation, we think that the PPI lawsuits in the highest settlement tier will have a settlement value of $100,000 to $150,000. These will likely be mostly kidney failure lawsuits. Cases in the second tier may end up being worth $60,000 to $90,000. The lowest tier cases may have a value of $20,000 to $50,000.
Another Marker for Nexium Lawsuit Settlement Amounts
Another marker for predicting the average settlement amount in a Nexium lawsuit (or any PPI lawsuit) would be looking at other kidney injury lawsuits. Let’s take a look at a few our lawyers could find to help understand potential settlement compensation payouts.
- 2021, California: $500,000 Settlement. A 12-year-old boy came under a pediatrician’s care. The pediatrician was prescribed several medications, including albuterol, sulfasalazine, and dexamethasone. The boy suffered a bowel perforation, liver failure, sepsis, kidney failure, Stevens-Johnson Syndrome, nephrotoxicity, hyperbilirubinemia, and abdominal pain. He required an ileostomy pouch for a week. The boy’s foster parents alleged negligence against the pediatrician. They claimed he was prescribed contraindicated medications and failed to appreciate his symptoms. This case settled for $500,000.
- 2020, Pennsylvania: $1,000,000 Settlement. A 52-year-old woman came under a nursing home’s care. She suffered renal failure, worsening bedsores, malnutrition, dehydration, sepsis, pneumonia, and infections. The woman died from her injuries. Her family alleged negligence against the nursing home. They claimed its staff provided substandard care and failed to devise an appropriate care plan. This case settled for $1,000,000.
- 2020, South Carolina: $1,000,000 Settlement. A 59-year-old woman came under a nursing home’s care. She suffered renal failure. The woman now required lifelong dialysis. She died before the case closed. The woman’s family alleged negligence against the nursing home. They claimed its staff failed to appreciate her prior kidney transplant, discontinued her immunosuppressant medication, and provided substandard care. This case settled for $1,000,000.
- 2020, South Carolina: $750,000 Settlement. A 67-year-old man underwent a screening colonoscopy. He suffered abdominal cramps, rectal bleeding and spasms, confusion, weakness, and dark urine. The man presented to the physician who performed the colonoscopy. He received a primary care physician referral. Three days later, the man underwent an exploratory laparotomy. He was diagnosed with a perforated colon. The man underwent a colostomy. He developed acute liver failure, kidney failure, blood loss anemia, and metabolic acidosis. The man died the following week. His family alleged negligence against the physician. They claimed he failed to recognize colon perforation signs and delayed his treatments. This case settled for $750,000.
- 2020, Pennsylvania: $1,000,000 Settlement. A 67-tear-old man underwent chemotherapy. He suffered an anaphylactic reaction. The man went into cardiac arrest. He developed kidney failure, anoxic brain damage, aspiration pneumonia, and sepsis. The man died from his injuries. His family alleged negligence against the hospital. They claimed its staff provided negligence care, failed to check his vital signs, timely stop the infusion, and timely treat anaphylaxis. This case settled for $1,000,000.
More Required Reading
- New 2023 study in JAMA Network Open finding an association between popular heartburn drugs, such as Nexium, Prilosec, and Prevacid, and acquiring drug-resistant infections.The study revealed an increased risk of acquiring extended-spectrum β-lactamase (ESBL) or carbapenemase-producing Enterobacterales infections in patients taking PPIs. Protonix, Zegerid, AcipHex, Dexilant, Vimovo, and generic equivalents are included in the class of drugs. Another thing you may want to discuss with your doctor.
 Lawsuit Information Center
Lawsuit Information Center